Clinical Trials Should Be Scientifically Sound and Described in a
26 A trial should be conducted in compliance with the protocol that has received prior independent ethics committee approval 27 The medical care given to and medical decisions made on behalf. Clinical trials should be scientifically sound and described in a clear detailed protocol.
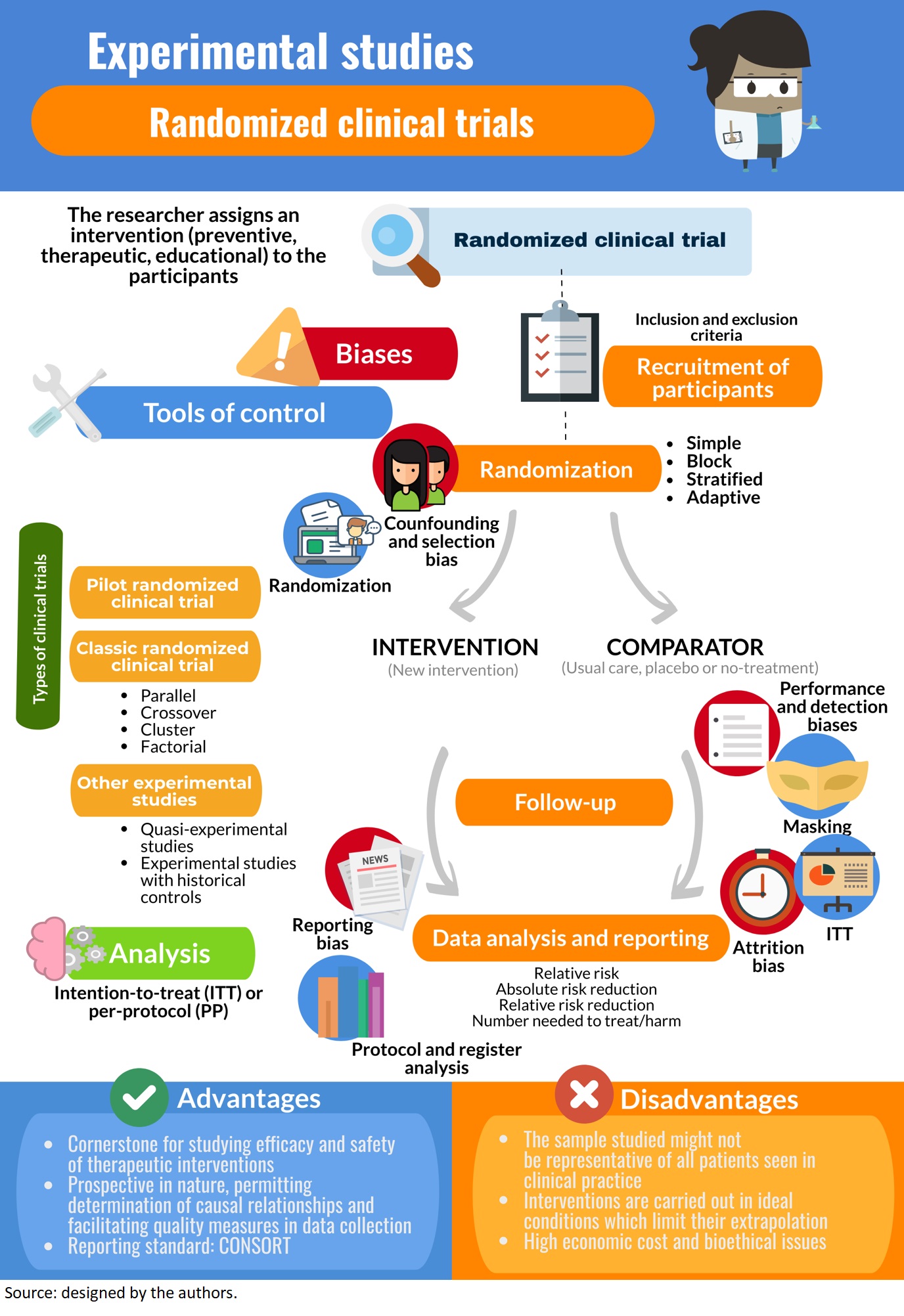
General Concepts In Biostatistics And Clinical Epidemiology Experimental Studies With Randomized Clinical Trial Design Medwave
25 Clinical trials should be scientifically sound and described in a clear detailed protocol.

. A trial should be conducted in compliance with the protocol that has received prior Institutional Review Board IRBIndependent Ethics Committee IEC approval. Ensure that clinical trials are scientifically sound and described in a clear detailed Protocol ICH-GCP E6 25. A trial should be conducted in compliance with the protocol that has received prior institutional review board IRBindependent ethics committee IEC approvalfavourable opinion.
27 The Subjects medical care should always be the responsibility of a qualified physician. Clinical trials should be scientifically sound and described in a clear detailed protocol. Clinical trials should be scientifically sound and described in a clear detailed protocol.
See also NYU SOPs Section 3711 for non-ICH-GCP requirements. On Clinical Trial and Good Clinical Practice Phase 2 4 7 Health Products and Food Branch ICH GCP. Clinical trials should be scientifically sound and described in a clear detailed protocol.
A trial should be conducted in compliance with the protocolthat has received. Identifies as a principle of good clinical practice that clinical trials should be scientifically sound 7 Other international guidance documents include similar. Clinical trials should be conducted in accordance with the ethical principles that are consistent with GCP and the applicable regulatory requirements and that have their origin in the Declaration of _____.
Clinical trials should be scientifically sound and described in a clear detailed protocol. Investigators and research staff provide all the disclosures and follow the requirements pertaining to consent covered by ICH-GCP. Clinical trials should be scientifically sound described in a clear and detailed protocol.
13 Principles of GCP 13 Principles of GCP 5. Compliance with the study protocol A trial should be conducted in compliance with the protocol that has received prior. The rights safety and well-being of trial participants are of paramount importance and these should be preserved by obtaining informed consent and maintaining confidentiality.
25 Clinical trials should be scientifically sound and described in a clear detailed protocol. Basic Elements of Informed Consent. A trial should be conducted in compliance with the protocol that has received prior institutional review board IRBindependent ethics committee IEC approvalfavourable opinion.
A trial should be conducted in compliance with the protocol that has received prior institutional review board IRBindependent ethics committee IEC approvalfavourable opinion. A trial should be conducted in compliance with the protocol that has received prior institutional review board IRBindependent ethics committee IEC approvalfavorable opinion. The clinical trial should be conducted in compliance with the study protocol.
26 A trial should be conducted in compliance with the protocol that has received prior institutional review board IRBindependent ethics committee IEC. A trial should be conducted in compliance. Clinical trials should be scientifically sound and clearly described in the study protocol.
Clinical trials should be scientifically sound and described in a clear detailed protocol. Clinical trials should be scientifically sound and described in clear detailed protocol. Compliance with the study protocol A trial should be conducted in compliance with the protocol that has received.
Approach to trial quality Clinical trials should be scientifically sound and described in a clear detailed protocol E6 25 Each individual involved in conducting a trial should be qualified by education training and experience to perform his or her respective tasks E6 28. Medicinal product should be adequate to support the proposed clinical trial. Clinical trials should be scientifically sound and described in a clear detailed protocol.
A trial should be conducted in compliance with the protocol that has received prior institutional review board IRBindependent ethics committee IEC approvalfavorable opinion. 26 A trial should be conducted according to the protocol having received prior IRB approval. All clinical trials should be conducted in accordance with ethical principles sound scientific evidence and clear detailed protocols.
Good quality trials Clinical trials should be scientifically sound and be described in a clear detailed protocol. In addition the IRB will determine that available information nonclinical and clinical on an investigational product is adequate to support a proposed clinical trial and that the study is scientifically sound and described in a clear detailed protocol. Adequate to support the proposed clinical trial.
Clinical trials should be scientifically sound and described in a clear detailed protocol. The benefits of conducting trials should outweigh the risks. 25 Clinical trials should be scientifically sound and described in a clear detailed protocol.
Clinical trials should be scientifically sound and described in a clear detailed protocol. Product should be adequate to support the proposed clinical trial. A trial should be conducted in compliance with.
To be approved clinical trials must satisfy the requirements described in HRPP policy Review of Research by the Convened IRB. The clinical study must be approved by an Institutional Review Board IRB a committee that has been formally designated to approve monitor and review biomedical and behavioral research.

Whitehall Training Want A Handy Guide Explaining Gcp Guidelines And Recent Changes Here S A Snapshot Of The Gcp Principles Including Recent Amendments Click The Link To Buy Now Goo Gl Bk42i2 Facebook

Considerations For Regulatory Oversight Of Clinical Trials In The Covid 19 Pandemic 5 June 2020

No comments for "Clinical Trials Should Be Scientifically Sound and Described in a"
Post a Comment